What Is The Density Of A Cube With 25G Mass And 125 Cm³ Volume?
How To Find Density, Mass, And Volume
Keywords searched by users: What is the density of a cube that has a mass of 25 grams and a volume of 125 cm³ what is the density of a piece of wood with a mass of 25 kg and a volume of 0.0385 m3, what is the volume of nickel 0.56 kg with density of 8.90 g cm, at 15 c air has a density of approximately 1.23kg m 3 calculate the density in g/cm^3, how to find mass given density, how to calculate volume with density and mass, how to find density of a liquid, density formula, how to find density without volume
What Is The Density Of An Object Having A Mass Of 25G And A Volume Of 5Cm?
The density of an object is a measure of how tightly its mass is packed within a given volume. To calculate density, you can use the formula: Density (D) = Mass (M) divided by Volume (V), expressed as D = M/V. In this specific case, we have an object with a mass of 25 grams and a volume of 5 cubic centimeters (cm³).
To find the density, simply divide the mass by the volume: D = 25g / 5cm³, which equals 5 grams per cubic centimeter (g/cm³).
It’s worth noting that for objects with irregular shapes, like cubes, you can calculate their volume using the formula for a rectangular shape: Volume = Length (L) x Width (W) x Height (H). For example, if you have a cube with sides each measuring 3 centimeters (cm), you can find its volume by calculating 3cm x 3cm x 3cm, which equals 27 cubic centimeters (cm³).
How Do You Find The Density Of A Cube?
To determine the density of a cube, you should follow a straightforward process. Firstly, calculate the volume of the cube by multiplying its side length by itself three times (V = side length × side length × side length). Secondly, place the cube on a scale to measure its mass accurately. Finally, obtain the density of the cube by dividing its volume by its mass (density = mass / volume). This calculation allows you to quantify how tightly packed the material is within the cube. For example, if you were to find the density of a 3 cm-sided cube with a mass of 27 grams, you would perform the calculation as follows: V = 3 cm × 3 cm × 3 cm = 27 cm³, and then density = 27 g / 27 cm³ = 1 g/cm³. This result indicates that the cube has a density of 1 gram per cubic centimeter, providing valuable information about its composition or material type.
Categories: Share 65 What Is The Density Of A Cube That Has A Mass Of 25 Grams And A Volume Of 125 Cm³
See more here: taomalumdongtien.net
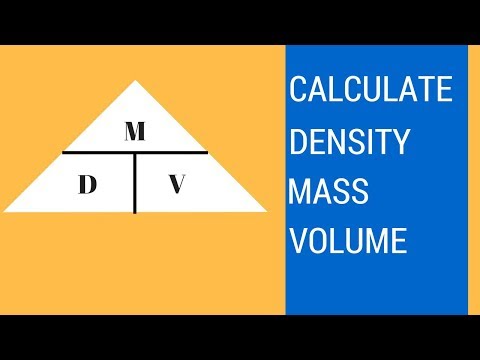
The density of an object is defined to be its mass divided by the volume it occupies. For this problem, the mass of the cube was given to be 25 g while its volume is 125 cm³. Thus, we simply divide 25 g by 125 cm³ to get the object’s density. We then calculate that the cube has a density of 0.2 g/ cm³.00119 g/cm3 Page 2 Density = Mass (g) divided by Volume (mL or cm3) → D = M/V. The mass of an object is 25g. The volume of an object is 5 cm3. D = 25g/5cm3 = 5 g/cm3. Volume of a rectangular shaped object/cube = Length x Width x Height (L x W x H). Example – A cube has a length of 3 cm (Note – a cube has equal sides). …You would first need to calculate the volume by multiplying 3 by 3 by 3 (V = 3 * 3 * 3). You would also need to put the cube on a scale to measure its mass. Last of all, you would divide your volume by your mass to get the density of the cube (p = m / v).
Learn more about the topic What is the density of a cube that has a mass of 25 grams and a volume of 125 cm³.
- What is the density of a cube that has a mass of 25 g … – Brainly
- Density Practice Problems
- How to Calculate Density | Formula, Units & Examples – Study.com
- What is the density of an object having a mass of 8.0 g and a volume of 25 …
- What is the density of a piece of wood that has a mass of 25.0g … – Socratic
- What is the Density of Water? – Factors, Experiment, Temperature Scales …