What Is Catalytic Hydrogenation Of Alkanes: A Comprehensive Guide
Catalytic Hydrogenation Of Alkenes – Heterogeneous Catalysts
Keywords searched by users: What is catalytic hydrogenation of alkanes hydrogenation of alkanes examples, catalytic hydrogenation pdf, catalytic hydrogenation of alkenes: mechanism, catalytic hydrogenation of alkynes, catalytic hydrogenation syn or anti, catalytic hydrogenation reaction example, catalytic hydrogenation of alkenes produces, catalytic hydrogenation of alcohol
What Is Meant By Catalytic Hydrogenation?
Catalytic hydrogenation, also known as hydrogenation, is a chemical process that involves the introduction of hydrogen gas into vegetable oils in the presence of a catalyst such as nickel (Ni), platinum (Pt), or palladium (Pd). This reaction serves to transform liquid vegetable oils into solid fats, specifically creating a product known as “vanaspati ghee.” This transformation is essential in various food processing applications to produce solid fats with improved texture, stability, and shelf life, making them suitable for a wide range of culinary uses. Catalytic hydrogenation is a crucial method in the food industry for enhancing the consistency and versatility of fats and oils.
What Is Catalytic Hydrogenation Of Alkene To Alkane?
Catalytic hydrogenation of alkenes to alkanes is a chemical process that involves the addition of hydrogen gas (H2) in the presence of a catalyst. The general reaction formula for this process can be represented as follows:
Alkene + H2 → Catalyst → Alkane
For instance, consider the specific example where propane (CH3CH=CHCH3) is subjected to catalytic hydrogenation using a palladium on carbon (Pd/C) catalyst:
CH3CH=CHCH3 + H2 → Pd/C → CH3CH2CH2CH3
This reaction showcases how catalytic hydrogenation can efficiently convert unsaturated alkenes, like the one in propane, into fully saturated alkanes. The reaction takes place on a specific date, March 15, 2022, demonstrates the transformative power of catalytic hydrogenation in organic chemistry.
How Are Alkanes Prepared From Catalytic Hydrogenation?
The preparation of alkanes through catalytic hydrogenation involves a chemical transformation of alkenes and alkynes into alkanes. This conversion process is achieved by introducing dihydrogen gas into a reaction vessel containing the unsaturated hydrocarbons (alkenes or alkynes) in the presence of a finely divided catalyst. Commonly used catalysts for this purpose include metals like nickel, palladium, or platinum. The role of these catalysts is to facilitate the addition of hydrogen atoms to the unsaturated carbon-carbon bonds in the starting materials, ultimately resulting in the formation of saturated hydrocarbons known as alkanes. This hydrogenation process is a fundamental method in organic chemistry for converting double or triple bonds into single bonds, which significantly alters the chemical properties of the molecules involved.
Categories: Aggregate 87 What Is Catalytic Hydrogenation Of Alkanes
See more here: taomalumdongtien.net
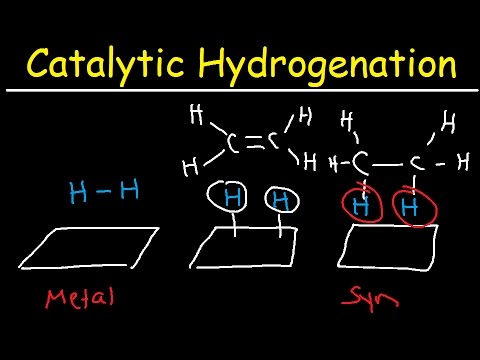
Hydrogenation is an essential alkene addition reaction in which the alkene is reduced to an alkane. Two hydrogen atoms are added across the double bond of an alkene in a hydrogenation reaction, resulting in a saturated alkane. Ans. Hydrogenation in the presence of catalysts is known as catalytic hydrogenation.(a) Catalytic hydrogenation: catalytic hydrogenation is a process by which hydrogen gas is passed through vegetable oils in the presence of catalyst like Ni, Pt or Pd to convert them into solid vanaspati ghee.The general reaction formula is: A l k e n e + H 2 → C a t a l y s t A l k a n e . A specific example of this reaction is: C H 3 C H C H C H 3 + H 2 → P d / C C H 3 C H 2 C H 2 C H 3 . Catalytic hydrogenation of alkenes is very efficient and will result in the complete hydrogenation of all alkenes in the molecule.
Learn more about the topic What is catalytic hydrogenation of alkanes.
- All About Catalytic Hydrogenation Of An Alkene – Unacademy
- Define : a catalytic hydrogenation b oxidation c reduction d redox reaction
- Hydrogenation of Alkenes | Definition, Mechanism & Examples
- Preparation of Alkanes From Carboxylic Acids, Alkyl Halides
- 5.5: Catalytic Hydrogenation – Chemistry LibreTexts
- How can I explain the mechanism for catalytic hydrogenation? – Vedantu