What Is The Effect Of Temperature On Henrys Constant: Exploring The Relationship
Pressure And Gas Solubility (Henry’S Law)
Keywords searched by users: What is the effect of temperature on Henry’s constant what is the effect of temperature on solubility of solid in liquid, applications of henry’s law, henry’s law solubility formula, state relation between henry’s constant and solubility of gas, state raoult’s law, the solubility of gases in water decreases with increasing temperature, does henry’s constant change with pressure, what is boiling point
Why Does Henry’S Constant Increase With The Increase In Temperature?
Understanding Henry’s Law and its Temperature Dependence
Henry’s law provides crucial insights into the solubility of gases in liquids. This fundamental principle states that the amount of a gas that dissolves in a liquid is directly proportional to the pressure of that gas above the liquid. However, it’s important to note that Henry’s constant, a key parameter in this equation, exhibits an intriguing relationship with temperature.
As temperature rises, the solubility of gases typically decreases in accordance with Henry’s law. This phenomenon occurs because gas molecules become more energetic and agitated at higher temperatures, making them less inclined to interact with the liquid phase. Consequently, as the temperature increases, the value of Henry’s constant actually increases, reflecting this inverse relationship. This concept highlights the intricate interplay between temperature and gas solubility, with important implications in various scientific and industrial applications.
Does Kh Change With Temperature?
Do changes in temperature affect a substance’s solubility in a liquid? Yes, they do. Generally, as the temperature of a liquid increases, the solubility of a gas in that liquid decreases. This phenomenon is often represented by the Henry’s Law constant, denoted as KH. Importantly, the KH value, which measures how much gas can dissolve in a liquid, tends to increase as the temperature of the system rises. In other words, higher temperatures typically lead to lower gas solubility and higher KH values, a key relationship to consider when studying the impact of temperature on the solubility of gases in liquids.
Is Henry’S Constant Inversely Proportional To Temperature?
Does Henry’s constant exhibit an inverse relationship with temperature? Inversely proportional variables behave in such a way that when one variable decreases, the other increases in the same proportion, and when one variable increases, the other decreases correspondingly. This stands in contrast to direct proportion, where both variables change in the same direction. In the case of Henry’s constant and temperature, a closer examination is needed to determine whether a decrease in temperature results in an increase or decrease in Henry’s constant, shedding light on their relationship.
Categories: Details 41 What Is The Effect Of Temperature On Henry’S Constant
See more here: taomalumdongtien.net
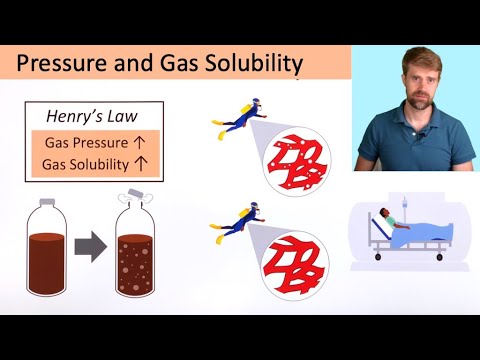
Here, Henry’s constant i.e is known to be inversely related to temperature. Hence, with the increases in temperature the value of Henry’s constant, and it in turn tends to decrease the solubility of the gas in a liquid.Explanation: According to Henry’s law, the solubility of a gas in a liquid is directly proportional to pressure of gas. Thus, the value of Henry’s constant increases with the increase in temperature.Solubility of a gas in liquid decreases with increase in temperature. KH value increases with the increase in temperature.
Learn more about the topic What is the effect of temperature on Henry’s constant.
- The effect of temperature on the solubility of a gas in a liquid is …
- Value of Henry’s constant KH ______. – India Site
- What is the effect of temperature on Henry’s law constant KH …
- Inversely Proportional – Definition, Formula and Examples – BYJU’S
- Henry’s Law – Statement, Formula, Constant, Solved Examples – BYJU’S
- How is Henry’s constant affected by change in temperature